Bromination of isobutane is a two step reaction – Bromination of isobutane is a two-step reaction mechanism that plays a crucial role in organic chemistry. This reaction involves the addition of bromine atoms to isobutane molecules, leading to the formation of various brominated products. Understanding the mechanism of this reaction is essential for comprehending its applications and industrial relevance.
The bromination of isobutane proceeds through a free radical chain mechanism, consisting of three distinct steps: initiation, propagation, and termination. During initiation, a bromine molecule homolyzes to generate bromine radicals. These radicals then react with isobutane molecules in the propagation step, abstracting a hydrogen atom and forming isobutyl radicals.
The isobutyl radicals subsequently react with bromine molecules, leading to the formation of brominated isobutane products and the regeneration of bromine radicals.
Bromination of Isobutane
Bromination is a significant reaction in organic chemistry that involves the addition of bromine atoms to an organic molecule. It is a versatile process used to introduce bromine into various organic compounds, leading to the synthesis of a wide range of products with diverse applications.
The bromination of isobutane is a two-step reaction that proceeds via a free radical mechanism. It serves as a model reaction to illustrate the principles and mechanisms of radical reactions in organic chemistry.
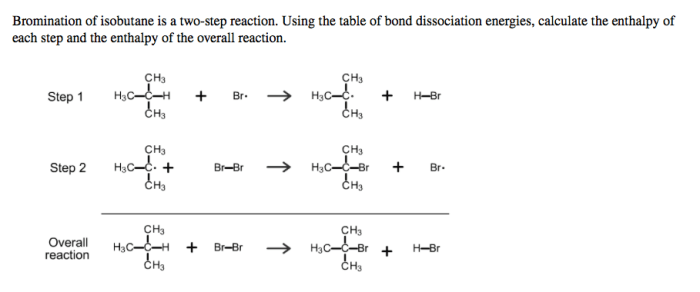
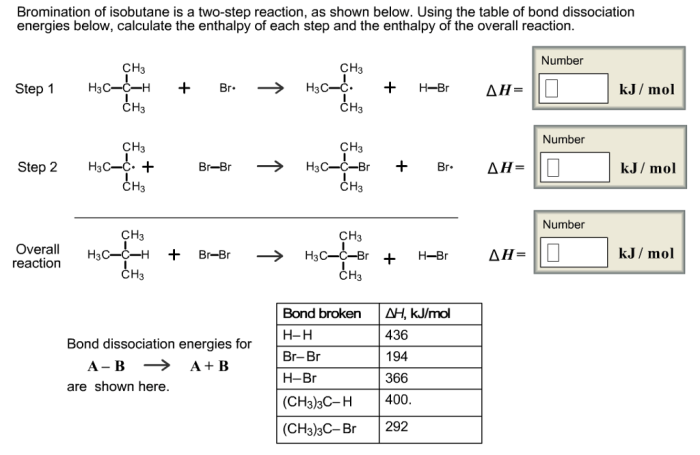
Mechanism of Bromination of Isobutane, Bromination of isobutane is a two step reaction
The bromination of isobutane proceeds through a two-step mechanism involving the following steps:
- Initiation:The reaction is initiated by the homolytic cleavage of a bromine molecule (Br 2) under the influence of light or heat, generating two bromine radicals (Br •).
- Propagation:The bromine radicals react with isobutane (C 4H 10) via hydrogen abstraction, producing isobutyl radicals (C 4H 9•) and hydrogen bromide (HBr). The isobutyl radicals further react with bromine molecules, leading to the formation of brominated isobutane products (C 4H 9Br) and additional bromine radicals, continuing the chain reaction.
- Termination:The reaction is terminated when two radicals combine to form a stable product, such as the dimerization of bromine radicals to form diatomic bromine (Br 2) or the combination of a bromine radical with an isobutyl radical to form 2-bromoisobutane (C 4H 9Br).
Two-Step Reaction Mechanism
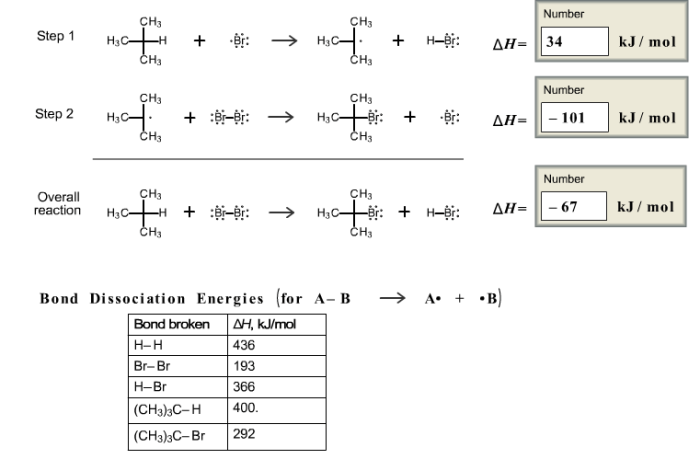
The bromination of isobutane is a two-step reaction that proceeds via a free radical mechanism. The first step involves the initiation of the reaction, where a bromine molecule homolytically cleaves to generate two bromine radicals. These radicals then react with isobutane via hydrogen abstraction, producing isobutyl radicals and hydrogen bromide.
The isobutyl radicals further react with bromine molecules, leading to the formation of brominated isobutane products and additional bromine radicals, continuing the chain reaction.
The second step involves the termination of the reaction, where two radicals combine to form a stable product, such as the dimerization of bromine radicals to form diatomic bromine or the combination of a bromine radical with an isobutyl radical to form 2-bromoisobutane.
The following reaction pathway illustrates the two-step mechanism of the bromination of isobutane:
- Initiation:Br 2→ 2 Br •
- Propagation:C 4H 10+ Br •→ C 4H 9•+ HBr
- C 4H 9•+ Br 2→ C 4H 9Br + Br •
- Termination:2 Br •→ Br 2
- C 4H 9•+ Br •→ C 4H 9Br
Experimental Procedures
Materials:
- Isobutane
- Bromine
- 2,2′-Azobis(2-methylpropionitrile) (AIBN) as a radical initiator
- Inert solvent (e.g., hexane or dichloromethane)
Equipment:
- Reaction flask
- Condenser
- Heating mantle
- Magnetic stirrer
Procedure:
- In a reaction flask equipped with a condenser, add isobutane, bromine, and AIBN.
- Heat the reaction mixture under reflux for several hours, while stirring.
- Allow the reaction mixture to cool to room temperature.
- Extract the organic layer with an inert solvent and wash with water.
- Dry the organic layer over anhydrous sodium sulfate.
- Filter the dried organic layer and concentrate it using a rotary evaporator.
- Analyze the product mixture using gas chromatography-mass spectrometry (GC-MS) or nuclear magnetic resonance (NMR) spectroscopy to identify and quantify the brominated isobutane products.
- Bromine is a toxic and corrosive substance. Handle it with care and wear appropriate personal protective equipment (PPE), including gloves, a lab coat, and a respirator.
- The reaction should be carried out in a well-ventilated area.
- Dispose of all chemicals and waste materials properly according to local regulations.
- Pharmaceuticals:Brominated isobutane is used as an intermediate in the synthesis of a variety of pharmaceuticals, such as anticonvulsants, sedatives, and muscle relaxants.
- Agrochemicals:Brominated isobutane is used as a fumigant and pesticide, particularly for the control of insects and pests in stored products.
- Specialty chemicals:Brominated isobutane is used as a flame retardant in plastics and textiles, and as a solvent in the electronics industry.
- The production of 2-bromoisobutane, which is used as an intermediate in the synthesis of the anticonvulsant drug phenobarbital.
- The production of bromochloromethane, which is used as a solvent and fumigant.
- The production of brominated flame retardants, which are used to enhance the fire resistance of plastics and textiles.
Safety Precautions:
Applications and Industrial Relevance
Brominated isobutane has a wide range of applications in various industries, including:
Some examples of commercial processes that utilize the bromination of isobutane include:
Question & Answer Hub: Bromination Of Isobutane Is A Two Step Reaction
What is the significance of the bromination of isobutane in organic chemistry?
The bromination of isobutane is a fundamental reaction in organic chemistry that serves as a model system for studying free radical mechanisms. It provides insights into the behavior of free radicals and their role in various chemical transformations.
How does the regioselectivity of the bromination of isobutane affect the product distribution?
The regioselectivity of the bromination of isobutane is influenced by the stability of the intermediate free radicals. The more stable free radical is formed preferentially, leading to the major product. This regioselectivity is crucial for controlling the outcome of the reaction and obtaining the desired brominated product.
What are the practical applications of the bromination of isobutane in industry?
The bromination of isobutane has numerous applications in industry. Brominated isobutane is used as an intermediate in the production of pharmaceuticals, agrochemicals, and other specialty chemicals. It is also employed as a solvent and a flame retardant.