Of the following which sublevel is filled last – The concept of sublevel filling order plays a pivotal role in determining the electronic structure of atoms, influencing their chemical properties and behavior. In this exploration, we delve into the fascinating world of atomic orbitals and uncover the intriguing order in which sublevels are filled, culminating in the identification of the last to fill.
Sublevel filling order is governed by the Aufbau principle and Hund’s rule, which guide the distribution of electrons within atomic orbitals. As we journey through the periodic table, we encounter elements that exhibit intriguing exceptions to this general order, providing insights into the complexities of atomic structure.
Sublevel Filling Order

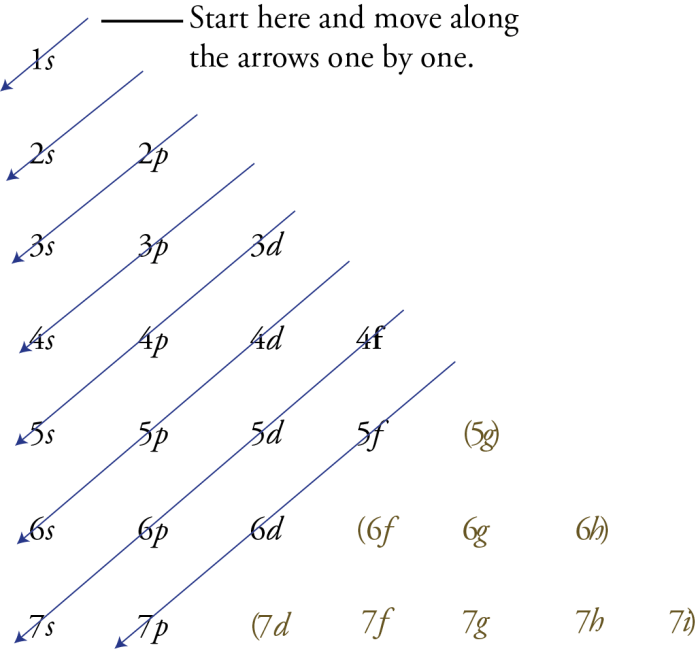
In atomic physics, the sublevel filling order refers to the sequence in which electrons occupy the available energy levels within an atom.
The Aufbau principle, named after Austrian physicist Wolfgang Pauli, governs the sublevel filling order. It states that electrons will occupy the lowest energy levels available before filling higher energy levels.
s, p, d, and f Sublevels
Atomic orbitals are classified into four types based on their shapes and orientations:
- s orbitals:Spherical in shape, with one maximum electron density region at the nucleus.
- p orbitals:Dumbbell-shaped, with two maximum electron density regions on either side of the nucleus.
- d orbitals:Complex shapes with four lobes, each containing two maximum electron density regions.
- f orbitals:Even more complex shapes with eight lobes, each containing two maximum electron density regions.
The sublevel filling order is s, p, d, and f, with each sublevel containing a maximum number of electrons:
- s: 2 electrons
- p: 6 electrons
- d: 10 electrons
- f: 14 electrons
Hund’s Rule, Of the following which sublevel is filled last
Hund’s rule, formulated by German physicist Friedrich Hund, states that when filling degenerate orbitals (orbitals with the same energy), electrons will occupy separate orbitals with the same spin before pairing up.
This rule ensures maximum stability for atoms and ions by minimizing electron-electron repulsion.
Exceptions to the Filling Order
Exceptions to the general sublevel filling order occur due to the stability of half-filled or fully filled orbitals:
- Chromium (Cr):[Ar] 3d 54s 1(instead of [Ar] 3d 44s 2)
- Copper (Cu):[Ar] 3d 104s 1(instead of [Ar] 3d 94s 2)
Applications of Sublevel Filling Order
Understanding sublevel filling order has practical applications in chemistry:
- Predicting chemical properties such as ionization energy and electron affinity
- Determining the electronic structure of molecules and solids
- Explaining the magnetic properties of atoms and ions
Quick FAQs: Of The Following Which Sublevel Is Filled Last
What factors determine the order of sublevel filling?
The order of sublevel filling is primarily governed by the Aufbau principle and Hund’s rule. The Aufbau principle dictates that electrons occupy orbitals of lowest energy first, while Hund’s rule states that electrons distribute themselves evenly across orbitals of equal energy, maximizing their spins.
Why do some elements exhibit exceptions to the general sublevel filling order?
Exceptions to the general sublevel filling order arise due to the stability of half-filled or fully filled orbitals. For instance, chromium (Cr) has an electron configuration of [Ar] 3d 54s 1, violating the expected filling order of 3d 44s 2. This exception can be attributed to the extra stability of the half-filled 3d 5configuration.
How does sublevel filling order impact chemical properties?
Sublevel filling order influences chemical properties such as ionization energy and electron affinity. Elements with electrons in higher energy sublevels tend to have lower ionization energies, making them more likely to lose electrons. Conversely, elements with electrons in lower energy sublevels have higher electron affinities, indicating a greater attraction for additional electrons.